Abstract
After elucidating a role for aberrant B Cell Receptor (BCR) signaling in chronic graft versus host disease (cGVHD) (Allen et al, Blood. 2014;123:2108), we showed that the proximal BCR molecule SYK is a viable therapeutic target in mice and patients (Flynn et al, Blood 2015; 125:4085. Poe et al, JCI Insight. 2018;3:e122430. NCT02611063). Increased SYK protein levels associate with enhance phosphorylation of SYK activation sites, leading to immediate BCR responsiveness in cGVHD. In mice that develop cGVHD manifestations after allogeneic bone marrow transplantation (BMT), BCR responsiveness is promoted by B Cell Activating Factor (BAFF) (Jia et al, Blood. 2021;137:2544). Instead of SYK protein levels decreasing upon engagement with surrogate antigen to regulate excessive BCR-signaling, SYK protein was maintained when BAFF and alloantigen were present. In the present study, we address a potential molecular mechanism underpinning SYK protein maintenance in cGVHD B cells.
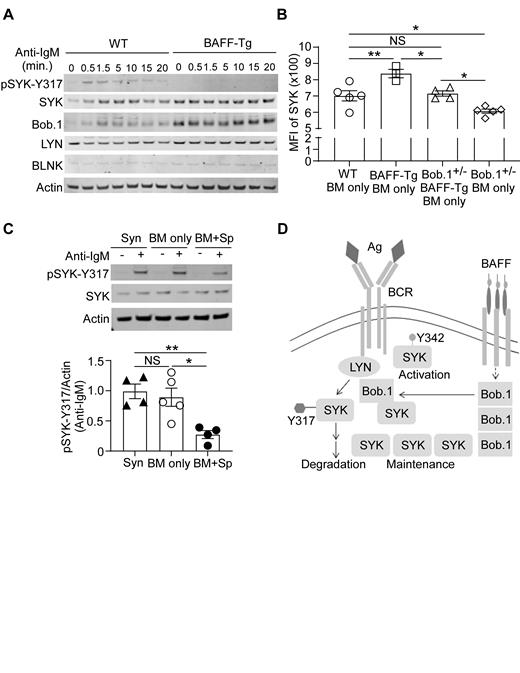
In an unbiased scRNAseq study examining purified B cells from active cGVHD patients, we found that Pou2af1 was significantly altered within a BCR-activated B cell subset (Poe et al, Blood. 2019; 134; Suppl1:874-874). Pou2af1 encodes B cell Oct binding factor 1 (Bob.1), a prime candidate for further study. Bob.1 is a transcriptional coactivator that is also able to bind to SYK and regulate SYK protein stability (Siegel et al, Cell. 2006; 125:761). To determine whether BAFF promoted increased Bob.1 expression we examined B cells from BAFF transgenic (Tg) mice. Consistent with a role for this molecule in blocking SYK degradation, Bob.1 protein levels were notably higher in BAFF-Tg B cells. Intracellular flow cytometry analyses of B cells at day 40 after BMT (cGVHD n=9 vs control n=5 mice) confirmed that Bob.1 is significantly higher in cGVHD mouse B cells (p=0.0002, data not shown). We then investigated whether phosphorylation of a SYK negative regulatory site after BCR-activation was affected by BAFF. Since tyrosine 317 (SYK-Y317) phosphorylation is known to initiate ubiquitination and degradation of SYK, we stimulated purified B cells through the BCR before preparing cell lysates and performing Western blot analyses to measure SYK-Y317 phosphorylation, total SYK, LYN, Bob.1 and BLNK. As shown in a representative blot in Fig 1A, phosphorylation of SYK-Y317 (pSYK-Y317) was significantly decreased in B cells from BAFF transgenic (BAFF-Tg) mice compared to those from wild type (WT) mice. These data suggest that BAFF promotes SYK through Bob.1.
Hypothesizing that Bob.1 is required to maintain SYK protein after allogeneic BMT, we employed Bob.1 knockout mice. After verifying that heterozygous mice (Bob.1 +/-) had lower Bob.1 protein levels and comparable B cell numbers to WT mice, we investigated the effect of Bob.1 reduction on SYK levels. Bob.1 +/- mice were crossed with BAFF-Tg mice and then SYK expression in B cells was determined using intracellular flow cytometry. We found that SYK protein levels were significantly increased in B cells exposed to a high BAFF environment and that SYK levels were significantly lower when Bob.1 protein was decreased (Fig 1B). These data demonstrate that Bob.1 is needed in vivo to maintain BAFF-mediated SYK protein maintenance after BCR engagement. Finally, we evaluated whether Bob.1 affected phosphorylation at SYK-Y317 in B cells from cGVHD mice. Splenic B cells were purified 41 days after BMT from recipients of B6 syngeneic (Syn), BM alone (BM only, control) or BM plus splenocytes (BM+Sp, cGVHD) and then stimulated with anti-IgM for 30 seconds before pSyk-Y317 and total SYK were detected by Western blot (Fig 1C). Using Image Studio Lite software, we normalized the intensity of pSyk-Y317 to actin in n=4 (Syn-BM+Sp), n=5 (BM only) and n=4 (BM+Sp) and found a significant decrease in pSyk-Y317 in cGVHD mice. We are currently investigating potential pathologic effects of Bob.1 after BMT in our mouse model and in patient cells.
In summary, we have now demonstrated that, Bob.1 is required to maintain SYK protein in B cells after allogeneic BMT in mice that develop cGVHD. Our data suggest that Bob.1 maintains the stability of BCR proximal SYK by blocking a known SYK degradation pathway (Fig 1D). Improving our understanding of Bob.1 and SYK protein homeostasis after HCT will impact how we implement SYK inhibitors in patients.
This work was supported the National Institutes of Health (NHLBI R01 HL 129061-06).
Sarantopoulos: Rigel: Other: Advisory Board.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal